by Glenn H. Sullivan, PhD and Guillermo E. Sanchez, PhD
Sustainable Nutritional International
Overview
Nutritional derivatives of the NutraLac® phytonutrient extraction technology were formulated into supplements for daily consumption by lactating mothers to determine the impact on the growth of their exclusively breastfed infants. The NutraLac® technology represents a pioneering opportunity to integrate the bioactive phytonutrients embodied in rice bran into a potent bioavailable nutritional food supplement derived from locally accessible byproducts of the rice milling process. This is particularly germane in nutrient deficit regions where lactating mothers and their infants are at high risk of life-long physical and mental stunting from chronic malnutrition, mainly during the first 1000 days of the child’s life.
Rice bran, a byproduct of the rice milling process worldwide, has been scientifically documented to be exceptionally high in bioactive phytonutrients which have potentially significant health benefits. However, the nutritional value of these phytonutrients generally has been unavailable in the human digestive system due to biological unavailability related to minimal enzymatic hydrolysis and modification. Our prior research found that the bioavailability of the latent bioactive phytonutrients in rice bran require enzymatic digestive processes uncommon to humans, thus the rice bran has generally been allocated to livestock feed after the rice milling process. However, the NutraLac® phytonutrient extraction process overcomes this impediment and significantly increases the nutritional bioavailability of these phytonutrient extracts in humans.
Sustainable Nutrition International, a 501c3 Organization, has been provided a royalty-free license for the utilization of the NutraLac® technology in humanitarian (non-commercial) program initiatives. It is the formulated phytonutrient derivatives of rice bran that represent the results of SNI’s 2013-14 field clinical trials attached. These trials were undertaken in collaboration with the Government of Guatemala.
Funding for our 2015-16 nutritional programs focused on expanded NutraLac® supplementation among diverse populations of nutritionally at-risk lactating mothers and their infants from 0 to 12 months of age in Central America.
NUTRITIONAL IMPACT OF NUTRALAC® CONSUMPTION IN LACTATING MOTHERS AND THEIR BREASTFED INFANTS IN EASTERN RURAL GUATEMALA
2012-2014
SUMMARY OF RESULTS
GENERAL INFORMATION AND INITIAL ANTHROPOMETRICS OF PARTICIPATING LACTATING WOMEN
| METRIC | ENGLISH | |
|---|---|---|
| Participants | 166 | |
| Communities | 17 | |
| Mean Age (Years) | 29.91 | |
| Mean Initial Height (Meters) | 1.47 | 4’10” |
| Mean Initial Body Mass Index | 22.5 ±2.6 | |
| Mean Initial Hematocrit (HCT) | 40% |
GENERAL INFORMATION AND INITIAL ANTHROPOMETRICS OF PARTICIPATING BREASTFED INFANTS
| Initial Participants | 153 |
| Final Participants | 129 |
| Males | 59 |
| Females | 70 |
| Mean Initial Age (Days) | 46±7. 9 |
| Mean Final Age (Mos.) | 6.18±0.31 |
| Mean Initial Weight (Kg) | 3..98 |
| Mean Initial Length (Cm) | 52.31 |
| Mean Initial Cephalic Perimeter (Cm) | 36.33 |
A total of 166 women were initially registered into the program and blood samples drawn from them to determine initial anemia prevalence. Due to several factors (reported inconsistency in taking the NutraLac® supplement, failure to show up at monthly anthropometric measurements, infants past the 65 days old threshold, etc.), a final group of 154 women and children were utilized as the initial group to be nutritionally assessed.
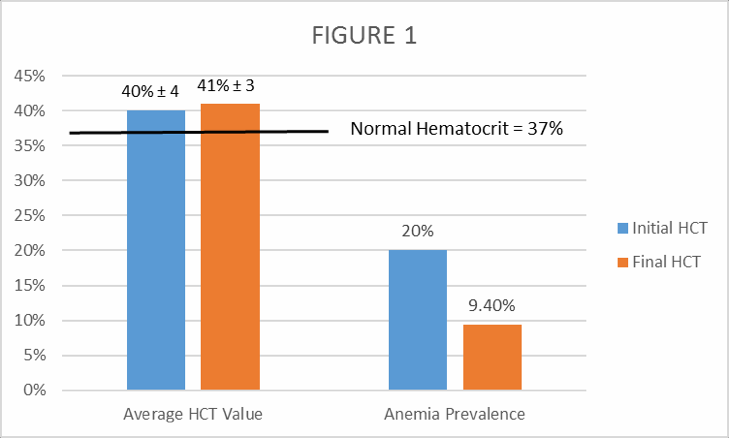
Figure 1. Average initial and final hematocrit (HCT) values and average initial and final anemia prevalence based on HCT results.
A normal hematocrit at Comapa’s altitude (1,300 meters above sea level) is 37% packed cells of the total volume in the blood sample. As shown in Figure 1, even though average HCT was very similar in initial and final readings, the prevalence of anemia (HCT<37% packed cell volume) was decreased from an initial 20% (1 in 5 women) to 9.4%.
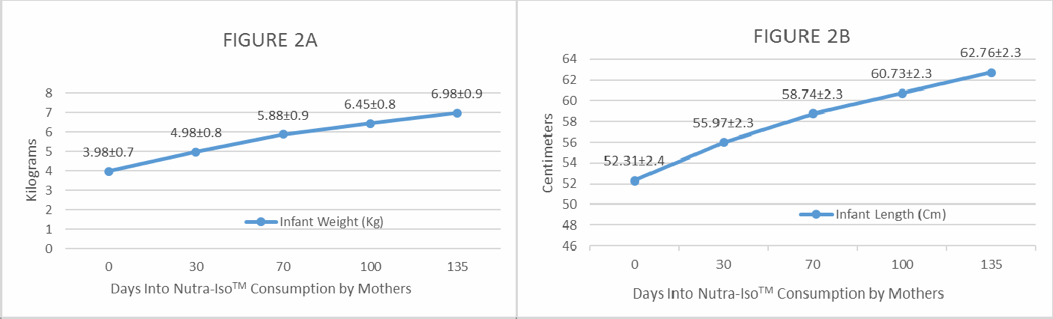
Figure 2. Monthly gains in weight (kg) and length (cm) of exclusively breastfed infants from mothers who took 40 daily grams of NutraLac® for a -day (4.5 months) period.
Figures 2A and 2B show that during the NutraLac® consumption period, infants gained an average of 667 grams (1.48 lbs.) per month in weight and gained an average of 2.32 cm a month in length. These are both healthy monthly gains, as well-fed infants at this age are expected to gain between 1.25 and 1.75 lbs. per month in weight and between 1.5 and 2.5 cm per month in length1. The average growth of 2.32 cm per month exhibited by the infants in the study was a very healthy growth rate.
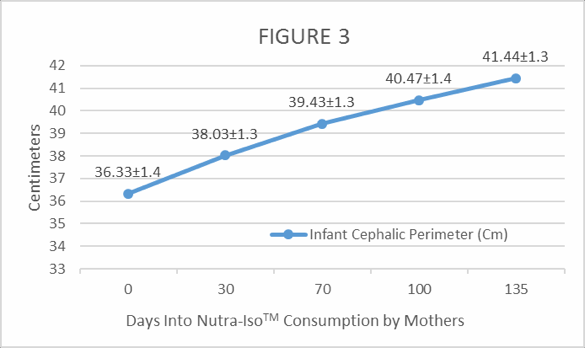
Figure 3. Monthly gains in Cephalic Perimeter (head circumference) growth of exclusively breast fed infants from mothers taking a 40-gram daily dose of NutraLac®.
As shown in Figure 3, the breastfed infants gained in average 1.15 cm per month in cephalic perimeter. A healthy head circumference gain in the first 6 months should range between 1 and 2 cm per month.2 All three anthropometric growth indicators taken from the exclusively breastfed infants showed healthy, normal growth rates.
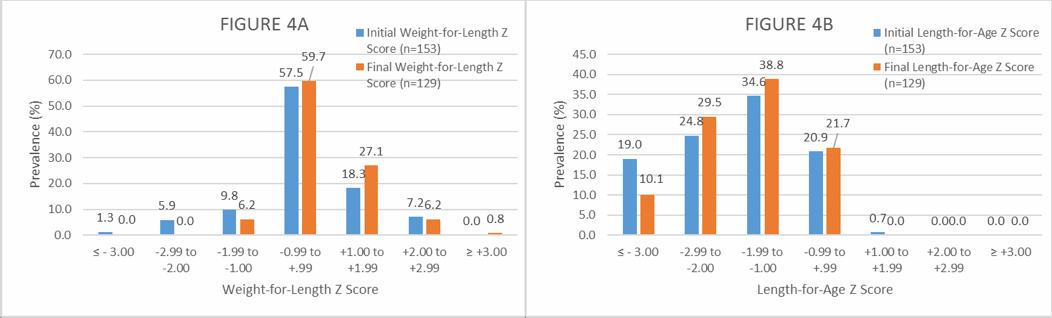
Figure 4. Distribution (%) of initial and final Weight-for-Length (4A) and initial and final Length-for-Age Z scores (4B) among the exclusively breastfed infants participating in the NutraLac® field clinical trials. Initial Z scores were obtained before the infants’ mothers started the daily consumption of NutraLac®, whereas the final Z scores were obtained at the end of the consumption window of 135 days.
As Figure 4A shows, the initial malnutrition prevalence rate (at an average age of 46 days) based on the weight-for-length Z score (acute malnutrition) shows that 5.9% of the children suffered from moderate acute malnutrition (MAM) and 1.3% exhibited severe acute malnutrition (SAM). At 6 months and the end of the consumption period, no children suffered from SAM or MAM. Figure 4B shows that at the beginning of the consumption period (at 46 days old), 44.2% of the infants fell in either the moderate (24.8%) or severe (19%) chronic malnutrition category. At the end of the consumption period, the chronically malnourished prevalence rate among infants was reduced to 39.6% (10.1% and 29.5%), while the severe chronic malnutrition rate was decreased by almost 50% from an initial prevalence rate of 19% to a final prevalence rate of 10.1%.
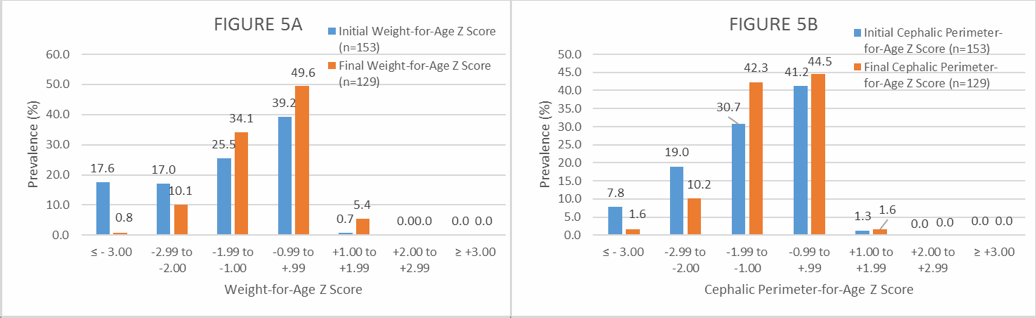
Figure 5. Distribution (%) of initial and final Weight-for-Age and (B) initial and final Cephalic Perimeter-for-Age Z scores among the exclusively breastfed infants participating in the NutraLac® field clinical trials. Initial Z scores were obtained before the infants’ mothers started the daily consumption of NutraLac®, whereas the final Z scores were obtained at the end of the consumption window of 135 days.
As shown in Figure 5, based on the weight-for-age malnutrition indicator, the weight-for-age malnutrition (Z score ≤ -2.0) was reduced from an initial prevalence of 34.6% to 10.9% among the infant population participating in the study. Regarding cephalic perimeter, at the beginning of the study the chronic malnutrition Z scores showed that 26.8% of the infant study group had a sub-normal growth rate (either moderate or severe). At the end of the consumption period, when infants were 6 months old, the sub-normal development of the head circumference had been decreased to 11.8% of the infant population.
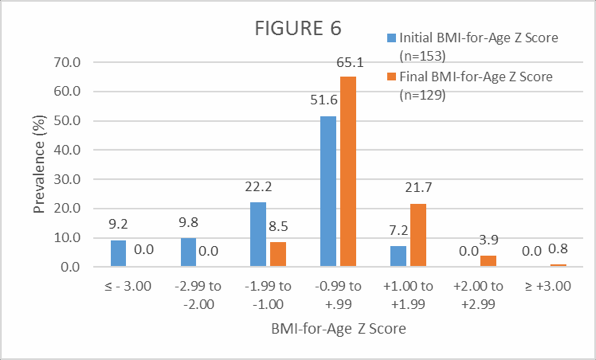
Figure 6. Distribution (%) of initial and final body mass index-for-age Z scores among the exclusively breastfed infants participating in the field NutraLac® trials. Initial Z scores were obtained before the infants’ mothers started the daily consumption of NutraLac®, whereas the final Z scores were obtained at the end of the consumption window of 135 days.
As shown in Figure 6, subnormal (indicator for malnutrition) BMI-for-age Z scores (≤ -2.0) were exhibited initially by 19% of the breastfed infant population in field clinical trials. After 135 days of NutraLac® consumption by their mothers, all the infants showed BMI-for-age Z scores greater than -2.0, meaning that all infants had recovered and surpassed the critical threshold for body mass index.
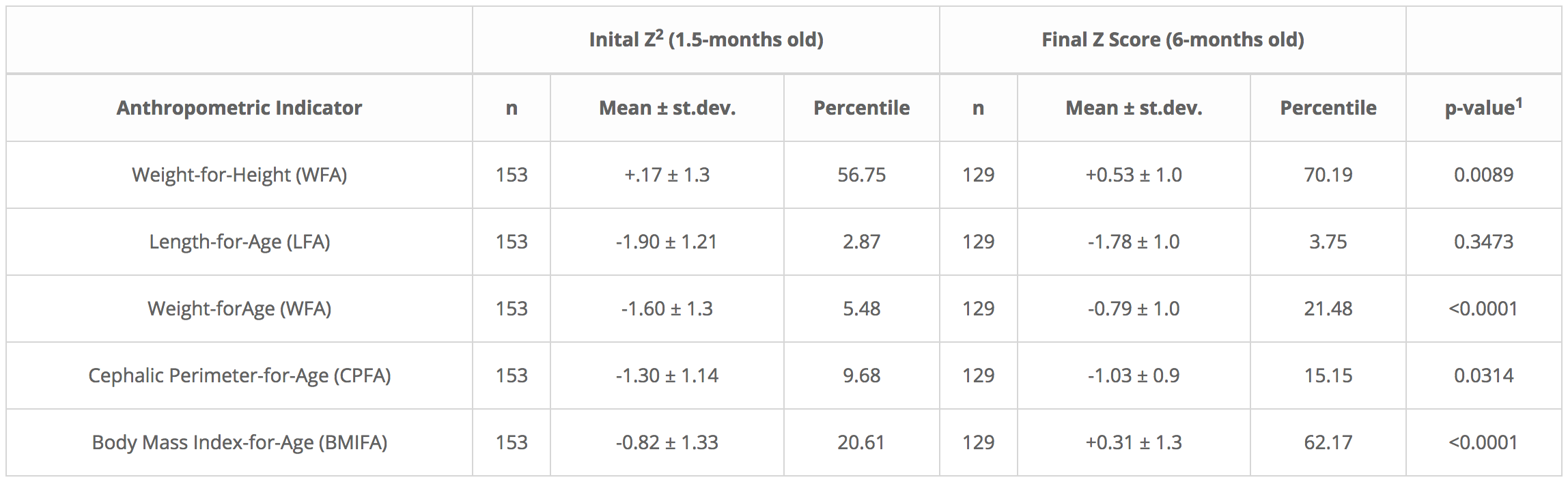
Table 1. Initial and final ‘Z’ scores and percentile results for five growth indicators of exclusively breastfed infants ranging from 1.5 to 6 months of age. Their mothers took a 40-g daily ration of a Rice Bran-Based Food Supplement (NutraLac) for a 4.5-month post-puerperium period.
Table 1
1 Student t Test for independent samples, INFOSTAT, version 2013. 2Z scores of anthropometric indicators, allow for comparison of results with the international child growth reference indicators from the World Health Organization (WHO). 2Z scores as determined by WHO-ANTHRO software, version 3.2.2.
Table 1 shows improvement in all 5 WHO growth indicators in the breastfed infants, following 135 days of NutraLac consumption by their mothers. As determined through a Student “t” test, three key indicators for infant growth, weight-for-height/length (WFH), weight-for-age (WFA) and Body Mass Index-for-age (BMIFA) exhibited highly statistically significant improvements (p<0.01), increasing 23.7%, 291.9%, and 202.0% respectively. The crucial preliminary indicator for brain growth, cephalic perimeter-for-age (CPFA) showed a 56.5% improvement, indicating that consumption of the bioactive rice-bran derived food supplement by the lactating mothers significantly (p<0.05) enhanced the head size in the breastfed infant study population. This is an important precursor to cognitive growth as the child matures. Table 1 also shows that the standard deviations were larger at the beginning of the field clinical trial, in contrast to the standard deviations at the end of the consumption period, when infants were 6 months old showing that Z-score average groupings were more compact at the end of the trial.
Figure 5. Distribution (%) of initial and final Weight-for-Age and (B) initial and final Cephalic Perimeter-for-Age Z scores among the exclusively breastfed infants participating in the NutraLac® field clinical trials. Initial Z scores were obtained before the infants’ mothers started the daily consumption of NutraLac® whereas the final Z scores were obtained at the end of the consumption window of 135 days.
As shown in Figure 5, based on the weight-for-age malnutrition indicator, the weight-for-age malnutrition (Z score ≤ -2.0) was reduced from an initial prevalence of 34.6% to 10.9% among the infant population participating in the study. Regarding cephalic perimeter, at the beginning of the study the chronic malnutrition Z scores showed that 26.8% of the infant study group had a sub-normal growth rate (either moderate or severe). At the end of the consumption period, when infants were 6 months old, the sub-normal development of the head circumference had been decreased to 11.8% of the infant population.

Figure 6. Distribution (%) of initial and final body mass index-for-age Z scores among the exclusively breastfed infants participating in the NutraLac® field clinical trials. Initial Z scores were obtained before the infants’ mothers started the daily consumption of NutraLac® whereas the final Z scores were obtained at the end of the consumption window of 135 days.
As shown in Figure 6, subnormal (indicator for malnutrition) BMI-for-age Z scores (≤ -2.0) were exhibited initially by 19% of the breastfed infant population in the field clinical trials. After 135 days of NutraLac® consumption by their mothers, all the infants showed BMI-for-age Z scores greater than -2.0, meaning that all infants had recovered and surpassed the critical threshold for body mass index.
Table 1. Initial and final ‘Z’ scores and percentile results for five growth indicators of exclusively breastfed infants ranging from 1.5 to 6 months of age. Their mothers took a 40-g daily ration of a Rice Bran-Based Food Supplement (NutraLac®) for a 4.5-month post-puerperium period.
Table 1
| Inital Z2 (1.5-months old) | Final Z Score (6-months old) |
|---|
| Anthropometric Indicator | n | Mean ± st.dev. | Percentile | n | Mean ± st.dev. | Percentile | p-value1 |
|---|---|---|---|---|---|---|---|
| Weight-for-Height (WFA) | 153 | +.17 ± 1.3 | 56.75 | 129 | +0.53 ± 1.0 | 70.19 | 0.0089 |
| Length-for-Age (LFA) | 153 | -1.90 ± 1.21 | 2.87 | 129 | -1.78 ± 1.0 | 3.75 | 0.3473 |
| Weight-forAge (WFA) | 153 | -1.60 ± 1.3 | 5.48 | 129 | -0.79 ± 1.0 | 21.48 | <0.0001 |
| Cephalic Perimeter-for-Age (CPFA) | 153 | -1.30 ± 1.14 | 9.68 | 129 | -1.03 ± 0.9 | 15.15 | 0.0314 |
| Body Mass Index-for-Age (BMIFA) | 153 | -0.82 ± 1.33 | 20.61 | 129 | +0.31 ± 1.3 | 62.17 | <0.0001 |
1 Student t Test for independent samples, INFOSTAT, version 2013. 2Z scores of anthropometric indicators, allow for comparison of results with the international child growth reference indicators from the World Health Organization (WHO). 2Z scores as determined by WHO-ANTHRO software, version 3.2.2.
Table 1 shows improvement in all 5 WHO growth indicators in the breastfed infants, following 135 days of NutraLac® consumption by their mothers. As determined through a Student “t” test, three key indicators for infant growth, weight-for-height/length (WFH), weight-for-age (WFA) and Body Mass Index for-age (BMIFA) exhibited highly statistically significant improvements (p<0.01), increasing 23.7%, 291.9%, and 202.0% respectively. The crucial preliminary indicator for brain growth, cephalic perimeter-for-age (CPFA) showed a 56.5% improvement, indicating that consumption of the bioactive rice-bran derived food supplement by the lactating mothers significantly (p<0.05) enhanced the head size in the breastfed infant study population. This is an important precursor to cognitive growth as the child matures. Table 1 also shows that the standard deviations were larger at the beginning of the field clinical trial, in contrast to the standard deviations at the end of the consumption period, when infants were 6 months old showing that Z-score average groupings were more compact at the end of the trial.
