Definition and Results from the Lactating Mothers and Breastfed Infants Study in Guatemala
Definition
Bioavailability relates to the rate and extent to which a nutrient (or drug) reaches the site of action within the body (i.e. the gastrointestinal absorption rate and efficacy). Most vitamins and minerals consumed orally are inorganic in nature and subsequently have low rates of gastrointestinal absorption and efficacy. Those nutritional supplements possessing high levels of phytonutrients and phytochemicals with pharmacological efficacy characteristics have high rates of gastrointestinal absorption and are subsequently bioactive (compounds that have an effect on tissue or body functions) for meeting specific health conditions.
NutraLac® is comprised of a complex of all natural vitamin, mineral and antioxidant isolates (nutraceutical phytonutrients) extracted from rice bran and possessing high pharmacological values, making NutraLac® highly bioavailable and efficacious in the human gastrointestinal tract. The naturally occurring phospholipids, phytosterols, tocotrienols and tocopherols in NutraLac® have been scientifically documented in playing important roles for significantly enhancing nutritional efficacy in mitigating certain chronic health conditions. The active natural nutraceutical ingredients in NutraLac® have been clinically documented to mitigate specific health conditions in human trials. NutraLac® has been awarded a U.S. Patent for reducing insulin resistance and oxidative stress in individuals concerned about pre-diabetic and type2 diabetic health conditions, with patents pending for mitigating nutritional deficiencies in lactating mothers and infants.
SUMMARY OF NUTRALAC® NUTRITIONAL SUPPLEMENTATION FOR LACTATING MOTHERS AND THEIR BREASTFED INFANTS
Infant chronic malnutrition affects 1 out of every 2 children under 5 years of age in Guatemala and 165 million children worldwide. The nutritional impact of NutraLac®, a rice bran extract (85%) supplemented with 7 natural vitamins and minerals (15%) to comply with World Health Organization (WHO) standards, was evaluated on lactating mothers (LM) and their exclusively breastfed infants (BI). The nutritional supplement (in a beverage presentation) was consumed daily by LM living in rural villages of the municipality of Comapa, in the Eastern Department of Jutiapa, republic of Guatemala in Central America. The nutritional intervention window had a duration of 4.5 month, starting when the BI were approximately 40-45 days old until the age of 6 months, at the end of the exclusive breastfeeding period. Determining the nutritional impact of NutraLac® on lactating mothers was one objective in this study, and adequate baseline weight data can only be obtained after the mother’s uterus has returned to a normal size (post-puerperium) and they have regained their normal pre-pregnancy weight. This regeneration period is known as puerperium and lasts approximately 40 days. The nutritional impact of NutraLac® on the BI was determined by length, weight and cephalic perimeter measurements, transformed to Z-scores and compared to WHO growth indicators and reference curves. The five growth indicators measured included: (1) weight-for-length (ZWL), (2) weight-for-age (ZWA), (3) length-for-age (ZLA), (4) cephalic perimeter-for-age (ZCPA) and (5) body mass index-for-age (ZBMIA). In LM´s the nutritional impact was determined by BMI prior to consumption and at the end of the 4.5-month intervention period. Anemia prevalence among LM´s was determined via micro-hematocrit test before and at finalization of the nutritional window.
Prior to the intervention and at 1.5 months old, 43% of BI´s were chronically malnourished and 7.2% suffered from moderate or severe acute malnutrition, while 25% and 18.4% showed delayed cerebral growth (ZCPA) and moderate or severe thinness (ZBMIA), respectively. At the end of the consumption period, no acute malnutrition was detected among the participants. Statistical analysis with student “T” test for independent samples determined that a highly significant improvement was observed among BI´s in the ZWL, ZWA and ZBMIA growth indicators at the end of the consumption window. A statistically significant improvement (p≤0.05) was observed in ZCPA, while ZLA exhibited only a slight improvement.
No statistically significant differences were found between BMI´s exhibited by LM´s before and at the end of the nutritional intervention; however, the prevalence of anemia was reduced from 21.2% to 11.4% among participants. LM´s also showed a statistically significant (p≤0.05) increase in their micro-hematocrit results and important increases in their breast milk production (anecdotal evidence). In conclusion, NutraLac® provided a significant nutritional contribution to both mothers and infants, when consumed daily by LM during the exclusive breastfeeding period. Since rice bran is present in almost all developing countries in the world, NutraLac® can have a potentially global impact, when this innovative rice bran stabilization and extraction technology is incorporated into worldwide chronic malnutrition mitigation and prevention programs.
DISCUSSION ON BIOACTIVITY AND BIOAVAILABILITY
Results show a statistically significant improvement in the exclusively breastfed infants’ nutritional status during the intervention window. Since maternal milk (MM) represented the sole food source that these infants received during the nutritional intervention, the improved growth exhibited by the BI’s was a direct result of either an increased consumption of MM, increased quality in the composition of the MM or a combination of both factors. The only significant change in the mothers’ diet was the daily addition of the NutraLac® supplement, as determined by 24-hour dietary recall interviews and food frequency surveys among LM´s. The claim that NutraLac®® consumption resulted in increased MM production is supported by the unanimous anecdotal evidence provided by the lactating mothers, who reported that between 20 and 30 days after having started to consume NutraLac®®, an increased MM production was evident; their breasts would fill with milk faster than prior to the nutritional intervention. As a result, they were able to increase the frequency and length of time at which their infants would breastfeed daily. These results, showing increased galactopoiesis, are clear evidence of the positive bioactivity of NutraLac®, which is generally defined as a compound or substance which triggers a response on living tissue or body1,2.
As Table 1 shows, statistically significant improvement in the nutritional status of the participating BI’s was initially detected in the weight-for-age results in the range between 30 days (Group 1) and 67 days (Group 2) into the intervention window. No significant difference in the nutritional status of BI´s was detected during the first 30 days of the study. It is interesting to observe that in the second half of the intervention period (from day 67 to day 135) the overall weight-for-age mean continued to improve among the group of infants but not at a statistically significant pace. This is understandable because infants had reached a weight-for-age status that placed them within the normal limits of the international WHO growth standards.
Table 1. Summary of T student test results for weight among participating breastfed infants in the NutraLac®® trials conducted in rural Eastern Guatemala, 2012- 2014. Infants were between 40 and 50 days old at the start of the nutritional intervention.
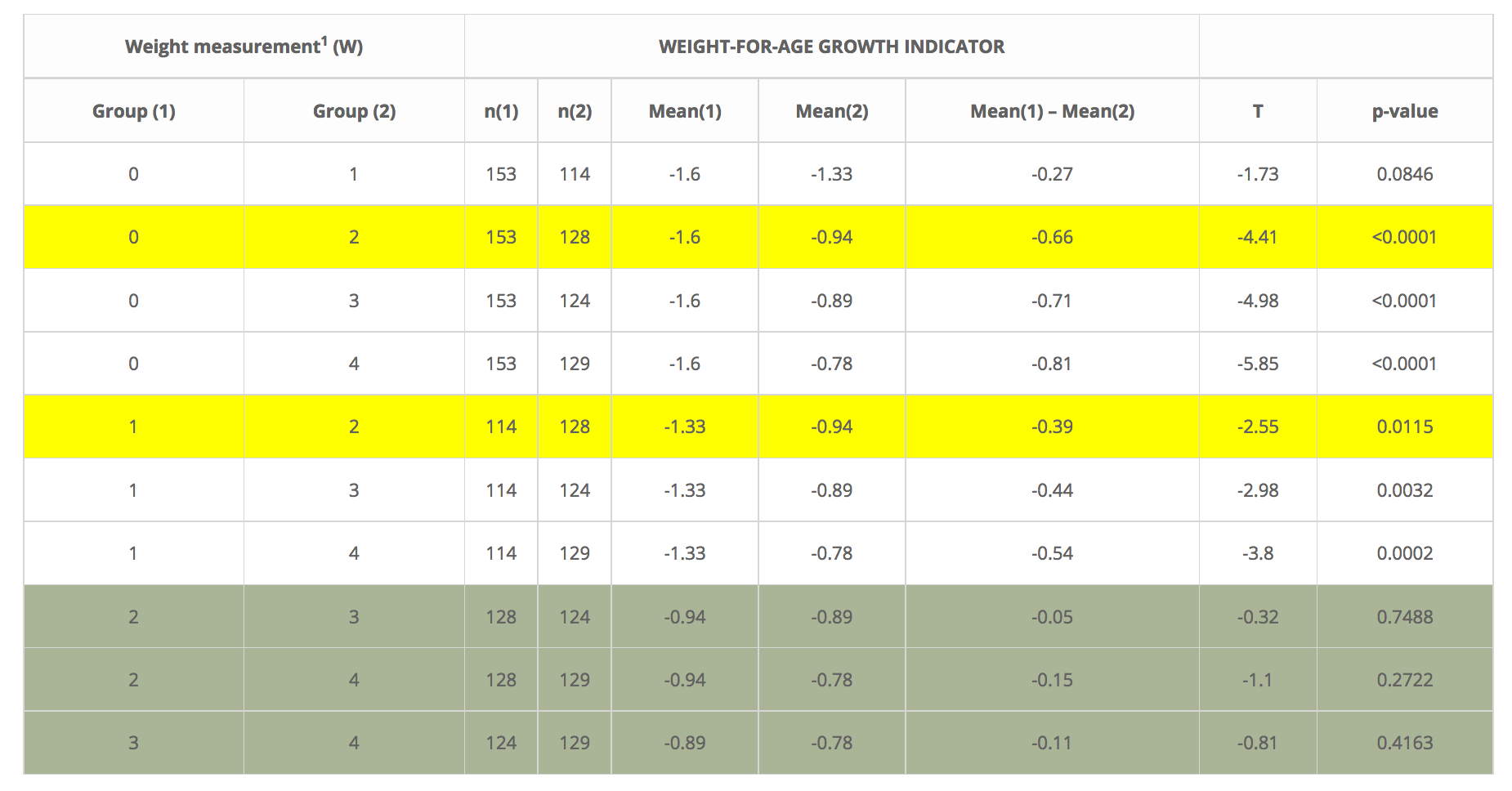
1 Exclusively breastfed infants were measured five times during the nutritional intervention window. Weight 0 (W0) was the baseline measurement prior to consumption of NutraLac® by their mothers, W1 was at 30 days after the start of the nutritional intervention and W2, W3, W4, and W5 were weight measurements taken at days 67, 97 and 135 (final) of the nutritional window.
The results mentioned above do seem to correlate with the observations reported by the lactating mothers in terms of increased MM production, which by their own account was markedly noticeable somewhere along the first 20 to 30 days after having started the daily consumption of NutraLac®. Therefore, it is understandable that an increased nutritional status among BI’s was detected approximately 30 days after the mothers were capable of increasing the frequency and duration of breastfeeding, given the increased MM production.
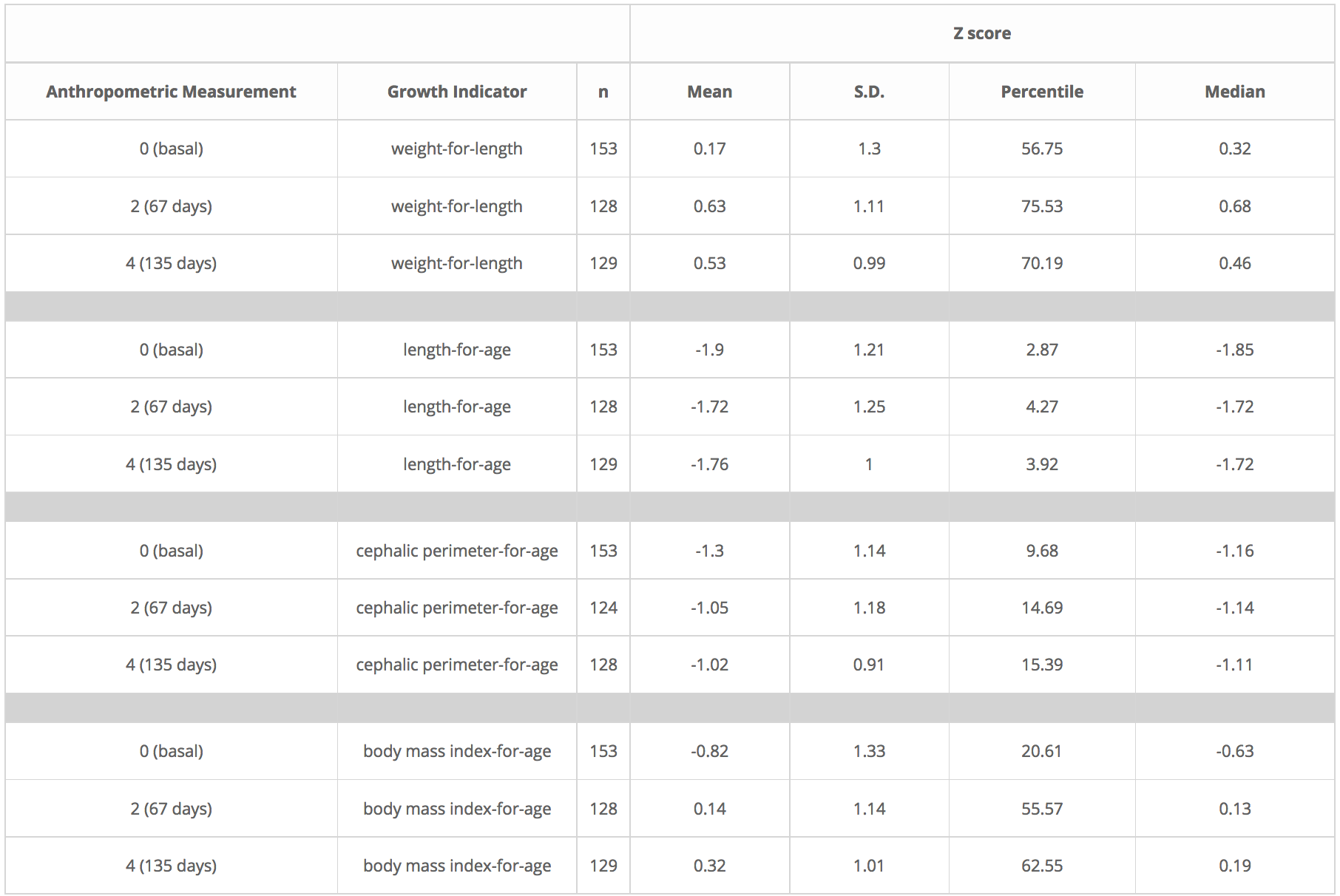
Similar results were obtained with the other 4 growth indicators, as Table 2 shows. With the exception of length-for-age, the 4 growth indicators showed improvement throughout the duration of the nutritional study, but only statistically significant during the first half of the nutritional intervention window (Table 3).
Table 2. Descriptive statistics for growth indicators of infants participating in the NutraLac®® clinical trials conducted in the dry corridor of Eastern Guatemala, 2012-2014.
After running a T student test to these 4 growth indicators, it was clear that the main effect of the nutritional intervention with NutraLac® came within the first 67 days of the trials (Table 3) as highly significant differences (p<0.0001) were found in the indicators weight-for-length, weight-for-age, and Body mass index-for-age, whereas significant differences (p<0.05) were found in the cephalic perimeter for age indicator, during the same time window.
The last indicator (Cephalic perimeter for age) is an important result. Scientific studies have clearly documented that the size of the cerebral circumference is directly correlated with brain growth and development. The results obtained in this study regarding brain development support the results from other studies in which delay in brain development can be reversed through timely nutritional intervention.
The fact that LM´s noticed an increased MM production within 20 to 30 days after the start of the intervention window seems to indicate that NutraLac® was readily assimilated by the participating mothers. Since current guidelines recommend exclusive breastfeeding during the first 180 days into the life of infants, a supplement like NutraLac® which evidences enhancement of MM galactopoiesis (lactogenesis stage 3) would prove to be immensely valuable for nutritional programs worldwide, as current intervention guidelines for the initial 6 months of a child’s life are entirely focused on exclusive breastfeeding and on training of LM´s in infant care and hygiene. While nutritional supplements that directly benefit lactating mothers are indeed available, there is little information regarding the existence of nutritional supplements that directly benefit the nutritional status of both the mother and the breastfed infant.
Table 3. Summary of T student test results for five growth indicators among breast-fed infants in the NutraLac® trials conducted in rural Eastern Guatemala, 2012- 2014. Infants were between 40 and 50 days old at the start of the nutritional intervention.
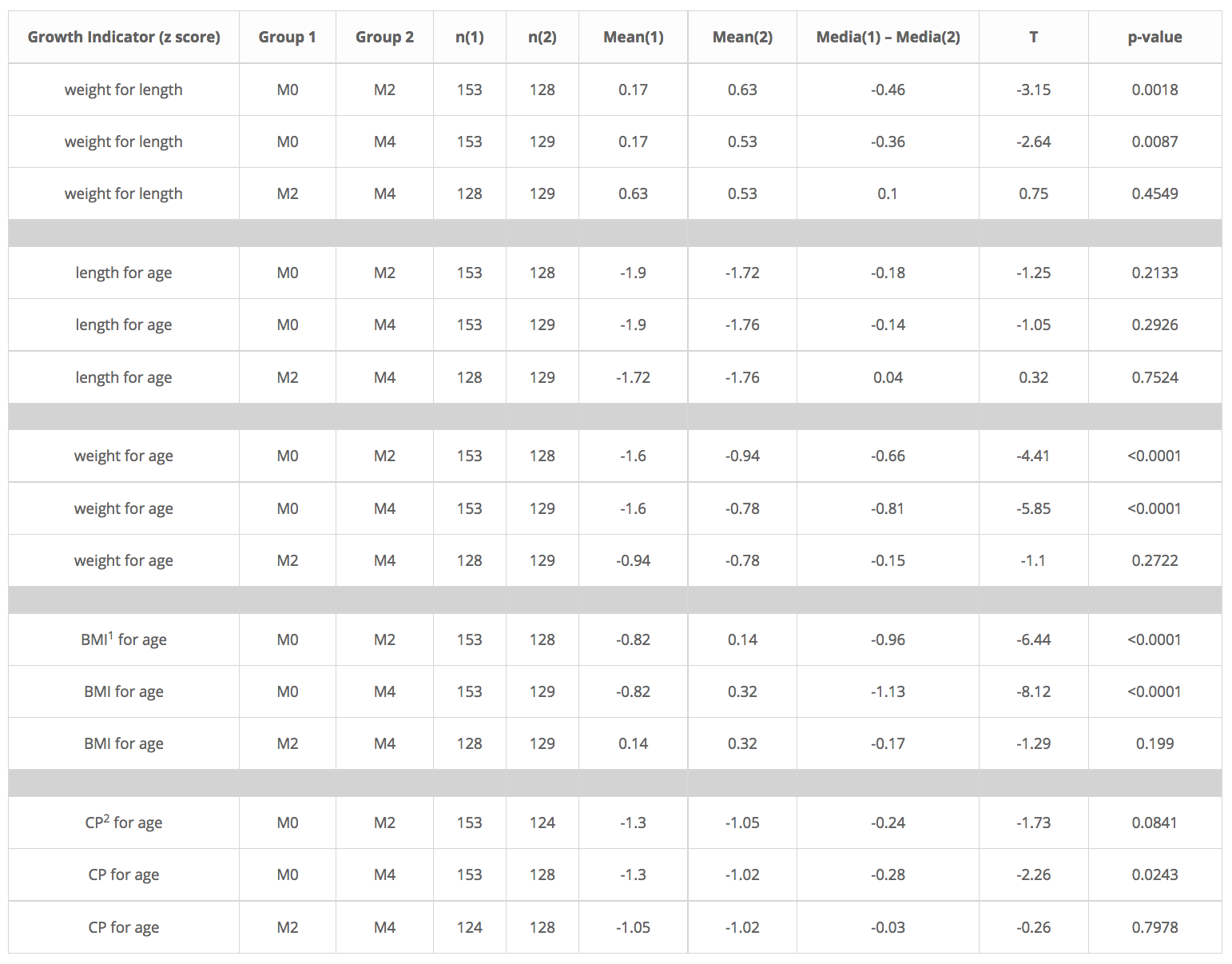
1 BMI:body mass index, 2 CP:Cephalic perimeter. Exclusively breastfed infants were measured five times during the nutritional intervention window. Measurement 0 (M0) was the baseline measurement prior to consumption of NutraLac® by their mothers, while M2 and M4 were weight growth measurements taken at days 67 and 135 (final) of the nutritional window.
Guillermo E. Sanchez, PhD3, Glenn H. Sullivan, PhD4 and Larry R. Miller, PhD5, August 17, 2016
1 Guaadaoui, A., Benaicha, S., Elmajdoub, N., Bellaoui, M., & Hamal, A. (2014). What is a bioactive compound? A combined definition for a preliminary consensus. International Journal of Nutrition and Food Sciences, 3(3), 174-179.
2 http://www.medicinenet.com/script/main/art.asp?articlekey=25616
3 ICADA Charitable Organization, Guatemala; (4) (5) Sustainable Nutrition International Charitable Organization (501c3), United States of America
Bioavailability and Bioactivity of NutraLac®
Definition and Results from the Lactating Mothers and Breastfed Infants Study in Guatemala
Definition
Bioavailability relates to the rate and extent to which a nutrient (or drug) reaches the site of action within the body (i.e. the gastrointestinal absorption rate and efficacy). Most vitamins and minerals consumed orally are inorganic in nature and subsequently, have low rates of gastrointestinal absorption and efficacy. Those nutritional supplements possessing high levels of phytonutrients and phytochemicals with pharmacological efficacy characteristics have high rates of gastrointestinal absorption and are subsequently bioactive (compounds that have an effect on tissue or body functions) for meeting specific health conditions.
NutraLac® is comprised of a complex of all natural vitamin, mineral and antioxidant isolates (nutraceutical phytonutrients) extracted from rice bran and possessing high pharmacological values, making NutraLac® highly bioavailable and efficacious in the human gastrointestinal tract. The naturally occurring phospholipids, phytosterols, tocotrienols and tocopherols in NutraLac® have been scientifically documented in playing important roles for significantly enhancing nutritional efficacy in mitigating certain chronic health conditions. The active natural nutraceutical ingredients in NutraLac® have been clinically documented to mitigate specific health conditions in human trials. NutraLac® has been awarded a U.S. Patent for reducing insulin resistance and oxidative stress in individuals concerned about pre-diabetic and type2 diabetic health conditions, with patents pending for mitigating nutritional deficiencies in lactating mothers and infants.
SUMMARY OF NUTRAISO® NUTRITIONAL SUPPLEMENTATION FOR LACTATING MOTHERS AND THEIR BREASTFED INFANTS
Infant chronic malnutrition affects 1 out of every 2 children under 5 years of age in Guatemala and 165 million children worldwide. The nutritional impact of NutraLac® a rice bran extract (85%) supplemented with 7 natural vitamins and minerals (15%) to comply with World Health Organization (WHO) standards, was evaluated on lactating mothers (LM) and their exclusively breastfed infants (BI). The nutritional supplement (in a beverage presentation) was consumed daily by LM living in rural villages of the municipality of Comapa, in the Eastern Department of Jutiapa, Republic of Guatemala in Central America. The nutritional intervention window had a duration of 4.5 months, starting when the BI was approximately 40-45 days old until the age of 6 months, at the end of the exclusive breastfeeding period. Determining the nutritional impact of NutraLac® on lactating mothers was one objective in this study, and adequate baseline weight data can only be obtained after the mother’s uterus has returned to a normal size (post-puerperium) and they have regained their normal pre-pregnancy weight. This regeneration period is known as puerperium and lasts approximately 40 days. The nutritional impact of NutraLac® on the BI was determined by length, weight and cephalic perimeter measurements, transformed to Z-scores and compared to WHO growth indicators and reference curves. The five growth indicators measured included: (1) weight-for-length (ZWL), (2) weight-for-age (ZWA), (3) length-for-age (ZLA), (4) cephalic perimeter-for-age (ZCPA) and (5) body mass index-for-age (ZBMIA). In LM´s the nutritional impact was determined by BMI prior to consumption and at the end of the 4.5-month intervention period. Anemia prevalence among LM´s was determined via micro-hematocrit test before and at finalization of the nutritional window.
Prior to the intervention and at 1.5 months old, 43% of BI´s were chronically malnourished and 7.2% suffered from moderate or severe acute malnutrition, while 25% and 18.4% showed delayed cerebral growth (ZCPA) and moderate or severe thinness (ZBMIA), respectively. At the end of the consumption period, no acute malnutrition was detected among the participants. Statistical analysis with student “T” test for independent samples determined that a highly significant improvement was observed among BI´s in the ZWL, ZWA and ZBMIA growth indicators at the end of the consumption window. A statistically significant improvement (p≤0.05) was observed in ZCPA, while ZLA exhibited only a slight improvement.
No statistically significant differences were found between BMI´s exhibited by LM´s before and at the end of the nutritional intervention; however, the prevalence of anemia was reduced from 21.2% to 11.4% among participants. LM´s also showed a statistically significant (p≤0.05) increase in their micro-hematocrit results and important increases in their breast milk production (anecdotal evidence). In conclusion, NutraLac® provided a significant nutritional contribution to both mothers and infants, when consumed daily by LM during the exclusive breastfeeding period. Since rice bran is present in almost all developing countries in the world, NutraLac® can have a potentially global impact, when this innovative rice bran stabilization and extraction technology is incorporated into worldwide chronic malnutrition mitigation and prevention programs.
DISCUSSION ON BIOACTIVITY AND BIOAVAILABILITY
Results show a statistically significant improvement in the exclusively breastfed infants’ nutritional status during the intervention window. Since maternal milk (MM) represented the sole food source that these infants received during the nutritional intervention, the improved growth exhibited by the BI’s was a direct result of either an increased consumption of MM, increased quality in the composition of the MM or a combination of both factors. The only significant change in the mothers’ diet was the daily addition of the NutraLac® supplement, as determined by 24-hour dietary recall interviews and food frequency surveys among LM´s. The claim that NutraLac® consumption resulted in increased MM production is supported by the unanimous anecdotal evidence provided by the lactating mothers, who reported that between 20 and 30 days after having started to consume NutraLac®, an increased MM production was evident; their breasts would fill with milk faster than prior to the nutritional intervention. As a result, they were able to increase the frequency and length of time at which their infants would breastfeed daily. These results, showing increased galactopoiesis, are clear evidence of the positive bioactivity of NutraLac®, which is generally defined as a compound or substance which triggers a response on living tissue or body1,2.
As Table 1 shows, statistically significant improvement in the nutritional status of the participating BI’s was initially detected in the weight-for-age results in the range between 30 days (Group 1) and 67 days (Group 2) into the intervention window. No significant difference in the nutritional status of BI´s was detected during the first 30 days of the study. It is interesting to observe that in the second half of the intervention period (from day 67 to day 135) the overall weight-for-age mean continued to improve among the group of infants but not at a statistically significant pace. This is understandable because infants had reached a weight-for-age status that placed them within the normal limits of the international WHO growth standards.
Table 1. Summary of T student test results for weight among participating breastfed infants in the NutraLac® trials conducted in rural Eastern Guatemala, 2012- 2014. Infants were between 40 and 50 days old at the start of the nutritional intervention.
| Weight measurement1 (W) | WEIGHT-FOR-AGE GROWTH INDICATOR |
|---|
| Group (1) | Group (2) | n(1) | n(2) | Mean(1) | Mean(2) | Mean(1) – Mean(2) | T | p-value |
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 153 | 114 | -1.6 | -1.33 | -0.27 | -1.73 | 0.0846 |
| 0 | 2 | 153 | 128 | -1.6 | -0.94 | -0.66 | -4.41 | <0.0001 |
| 0 | 3 | 153 | 124 | -1.6 | -0.89 | -0.71 | -4.98 | <0.0001 |
| 0 | 4 | 153 | 129 | -1.6 | -0.78 | -0.81 | -5.85 | <0.0001 |
| 1 | 2 | 114 | 128 | -1.33 | -0.94 | -0.39 | -2.55 | 0.0115 |
| 1 | 3 | 114 | 124 | -1.33 | -0.89 | -0.44 | -2.98 | 0.0032 |
| 1 | 4 | 114 | 129 | -1.33 | -0.78 | -0.54 | -3.8 | 0.0002 |
| 2 | 3 | 128 | 124 | -0.94 | -0.89 | -0.05 | -0.32 | 0.7488 |
| 2 | 4 | 128 | 129 | -0.94 | -0.78 | -0.15 | -1.1 | 0.2722 |
| 3 | 4 | 124 | 129 | -0.89 | -0.78 | -0.11 | -0.81 | 0.4163 |
1 Exclusively breastfed infants were measured five times during the nutritional intervention window. Weight 0 (W0) was the baseline measurement prior to consumption of NutraLac® by their mothers, W1 was at 30 days after the start of the nutritional intervention and W2, W3, W4, and W5 were weight measurements taken at days 67, 97 and 135 (final) of the nutritional window.
The results mentioned above do seem to correlate with the observations reported by the lactating mothers in terms of increased MM production, which by their own account was markedly noticeable somewhere along the first 20 to 30 days after having started the daily consumption of NutraLac®. Therefore, it is understandable that an increased nutritional status among BI’s was detected approximately 30 days after the mothers were capable of increasing the frequency and duration of breastfeeding, given the increased MM production.
Similar results were obtained with the other 4 growth indicators, as Table 2 shows. With the exception of length-for-age, the 4 growth indicators showed improvement throughout the duration of the nutritional study, but only statistically significant during the first half of the nutritional intervention window (Table 3).
Table 2. Descriptive statistics for growth indicators of infants participating in the NutraLac® clinical trials conducted in the dry corridor of Eastern Guatemala, 2012-2014.
| Z score |
|---|
| Anthropometric Measurement | Growth Indicator | n | Mean | S.D. | Percentile | Median |
|---|---|---|---|---|---|---|
| 0 (basal) | weight-for-length | 153 | 0.17 | 1.3 | 56.75 | 0.32 |
| 2 (67 days) | weight-for-length | 128 | 0.63 | 1.11 | 75.53 | 0.68 |
| 4 (135 days) | weight-for-length | 129 | 0.53 | 0.99 | 70.19 | 0.46 |
| 0 (basal) | length-for-age | 153 | -1.9 | 1.21 | 2.87 | -1.85 |
| 2 (67 days) | length-for-age | 128 | -1.72 | 1.25 | 4.27 | -1.72 |
| 4 (135 days) | length-for-age | 129 | -1.76 | 1 | 3.92 | -1.72 |
| 0 (basal) | cephalic perimeter-for-age | 153 | -1.3 | 1.14 | 9.68 | -1.16 |
| 2 (67 days) | cephalic perimeter-for-age | 124 | -1.05 | 1.18 | 14.69 | -1.14 |
| 4 (135 days) | cephalic perimeter-for-age | 128 | -1.02 | 0.91 | 15.39 | -1.11 |
| 0 (basal) | body mass index-for-age | 153 | -0.82 | 1.33 | 20.61 | -0.63 |
| 2 (67 days) | body mass index-for-age | 128 | 0.14 | 1.14 | 55.57 | 0.13 |
| 4 (135 days) | body mass index-for-age | 129 | 0.32 | 1.01 | 62.55 | 0.19 |
After running a T student test to these 4 growth indicators, it was clear that the main effect of the nutritional intervention with NutraLac® came within the first 67 days of the trials (Table 3) as highly significant differences (p<0.0001) were found in the indicators weight-for-length, weight-for-age and Body mass index-for-age, whereas significant differences (p<0.05) were found in the cephalic perimeter for age indicator, during the same time window.
The last indicator (Cephalic perimeter for age) is an important result. Scientific studies have clearly documented that the size of the cerebral circumference is directly correlated with brain growth and development. The results obtained in this study regarding brain development support the results from other studies in which delay in brain development can be reversed through timely nutritional intervention.
The fact that LM´s noticed an increased MM production within 20 to 30 days after the start of the intervention window seems to indicate that NutraLac® was readily assimilated by the participating mothers. Since current guidelines recommend exclusive breastfeeding during the first 180 days into the life of infants, a supplement like NutraLac® which evidences enhancement of MM galactopoiesis (lactogenesis stage 3) would prove to be immensely valuable for nutritional programs worldwide, as current intervention guidelines for the initial 6 months of a child´s life are entirely focused on exclusive breastfeeding and on training of LM´s in infant care and hygiene. While nutritional supplements that directly benefit lactating mothers are indeed available, there is little information regarding the existence of nutritional supplements that directly benefit the nutritional status of both the mother and the breastfed infant.
Table 3. Summary of T student test results for five growth indicators among breast-fed infants in the NutraLac® trials conducted in rural Eastern Guatemala, 2012- 2014. Infants were between 40 and 50 days old at the start of the nutritional intervention.
| Growth Indicator (z score) | Group 1 | Group 2 | n(1) | n(2) | Mean(1) | Mean(2) | Media(1) – Media(2) | T | p-value |
|---|---|---|---|---|---|---|---|---|---|
| weight for length | M0 | M2 | 153 | 128 | 0.17 | 0.63 | -0.46 | -3.15 | 0.0018 |
| weight for length | M0 | M4 | 153 | 129 | 0.17 | 0.53 | -0.36 | -2.64 | 0.0087 |
| weight for length | M2 | M4 | 128 | 129 | 0.63 | 0.53 | 0.1 | 0.75 | 0.4549 |
| length for age | M0 | M2 | 153 | 128 | -1.9 | -1.72 | -0.18 | -1.25 | 0.2133 |
| length for age | M0 | M4 | 153 | 129 | -1.9 | -1.76 | -0.14 | -1.05 | 0.2926 |
| length for age | M2 | M4 | 128 | 129 | -1.72 | -1.76 | 0.04 | 0.32 | 0.7524 |
| weight for age | M0 | M2 | 153 | 128 | -1.6 | -0.94 | -0.66 | -4.41 | <0.0001 |
| weight for age | M0 | M4 | 153 | 129 | -1.6 | -0.78 | -0.81 | -5.85 | <0.0001 |
| weight for age | M2 | M4 | 128 | 129 | -0.94 | -0.78 | -0.15 | -1.1 | 0.2722 |
| BMI1 for age | M0 | M2 | 153 | 128 | -0.82 | 0.14 | -0.96 | -6.44 | <0.0001 |
| BMI for age | M0 | M4 | 153 | 129 | -0.82 | 0.32 | -1.13 | -8.12 | <0.0001 |
| BMI for age | M2 | M4 | 128 | 129 | 0.14 | 0.32 | -0.17 | -1.29 | 0.199 |
| CP2 for age | M0 | M2 | 153 | 124 | -1.3 | -1.05 | -0.24 | -1.73 | 0.0841 |
| CP for age | M0 | M4 | 153 | 128 | -1.3 | -1.02 | -0.28 | -2.26 | 0.0243 |
| CP for age | M2 | M4 | 124 | 128 | -1.05 | -1.02 | -0.03 | -0.26 | 0.7978 |
1 BMI:body mass index, 2 CP:Cephalic perimeter. Exclusively breastfed infants were measured five times during the nutritional intervention window. Measurement 0 (M0) was the baseline measurement prior to consumption of NutraLac® by their mothers, while M2 and M4 were weight growth measurements taken at days 67 and 135 (final) of the nutritional window.
